Abstract
The length of telomeres, which cap the ends of linear chromosomes and provide genomic stability, is tightly regulated in adult stem cells. The telomere reserve in the stem cell population sets the replicative potential of its differentiated progeny. For this reason, abnormally short telomeres in stem cells restrict the number of cell divisions that their differentiated progenies can undergo, eventually resulting in stem cell depletion and tissue failure syndromes. Telomere biology disorders (TBDs) display a broad range of clinical features, age of onset, and severity, which are all correlated with the extent of abnormal telomere shortening. One such early-onset TBD is dyskeratosis congenita (DC), which is a bone marrow predisposition syndrome characterized by a mucocutaneous triad (oral leukoplakia, nail dystrophy, and abnormal skin pigmentation) as well as other conditions driven by premature tissue aging. The leading cause of death in DC patients is bone marrow failure and hematopoietic stem cell transplantation is the only definite intervention to restore hematopoiesis.
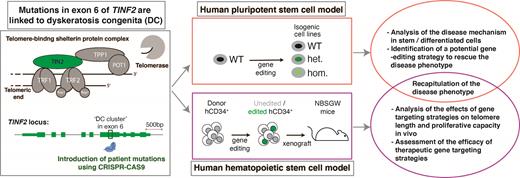
TINF2, which encodes the TIN2 protein, is mutated in 12% of patients and thereby the second most frequently altered gene in DC cases. TIN2 is a member of the shelterin protein complex bridging the double-strand binding shelterin proteins TRF1/TRF2, and the TPP1/POT1 heterodimer. Such interactions implicate a complex role of TIN2 in telomere length regulation: First, TIN2 stabilizes TRF1, which is a negative regulator of telomere length. Secondly, TPP1, which recruits telomerase, strictly requires TIN2 for telomere elongation and maintenance. TINF2-DC mutations are uniformly heterozygous and localize to a 30 amino acid coding stretch in exon 6 called the 'DC cluster'. TINF2-DC mutations usually arise de novo and result in an earlier disease onset, shorter telomeres, and a more severe manifestation compared to other heterozygous DC-causing mutations in genes such as TR and TERT, which encode the components of the telomerase enzyme.
How TINF2-DC mutations cause telomere shortening is unknown. Specifically, whether telomere shortening is caused by reduced telomerase action at telomeres or by degradation of telomeric DNA remains unresolved as studies using different model systems report contrasting results. The discrepancy could be attributed to differences in the model systems used in the studies, highlighting the need for a genetically trackable, primary preclinical human model system.
Here, we report the development of two novel endogenous, isogenic model systems to study TINF2-DC mutations. First, we generated human embryonic stem cells (hESCs) engineered to express the TINF2-DC T284R mutation from the endogenous locus, which recapitulated the short telomere phenotype observed in DC patients. Using this model, we identified a gene editing strategy that elongates telomeres in the mutant stem cells and eventually restores replicative potential of the differentiated cells. Next, we used a xenotransplantation model of donor-derived human hematopoietic stem cells (hHSCs) to test the effects of target gene modifications on telomere length and proliferative capacity in vivo. We demonstrate that our models robustly complement each other and offer direct insights into the disease mechanism as well as avenues to potential therapeutic approaches.
Bertuch: Elixirgen Therapeutics: Consultancy; ImmunityBio: Current equity holder in publicly-traded company; NIH/NCI,: Research Funding; DOD: Research Funding; Hyundai Hope on Wheels: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal